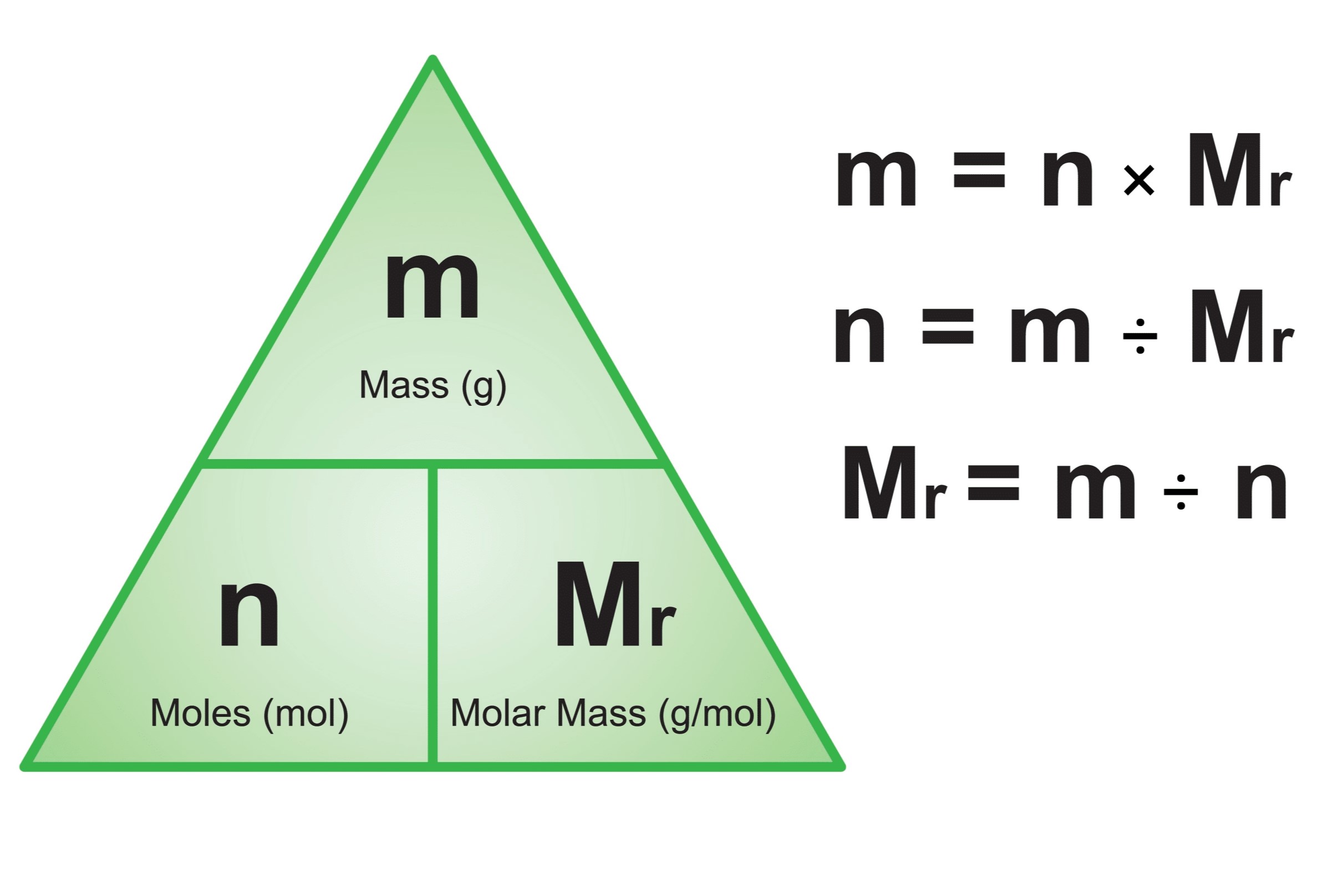
The vapour pressure of a solvent decreased by 10 mm of h g when a non-volatile solute was added to the solvent. What would be the mole fraction of each component in the solution? The vapour pressures of pure liquids a and b are 400 and 600 mm hg, respectively at 298 k. A solution of sucrose in water is labelled as 20 % w/w. The total vapour pressure of a 4 mole solution of n h 3 in water at 293 k is 50. 0 torr, the vapour pressure of pure water is 17. 0 torr at this temperature. A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? Chlorine is limiting reagent, in this case. If the density of solution is 1. 2 g ml −1, then what shall be the … 22 the mole fraction of the solute in one molal aqueous solution is 1)0. 027 2)0. 036 3)0. 018 4)0. 009 Calculate the mole fraction of glycerin c3h 5(oh)3 in a 100 g solution containing 33 g of glycerin, 60 g isopropyl alcohol and rest water. One mole of h 2o and one mole of co are taken in a 10 litre vessel and heated to 725 k. Applying henrys and raoults laws, … Define mole fraction. The energy required to dissociate 1 mole of iodine … What would be the mole fraction … Hence 0. 2-mole chlorine will react with 0. 1 moles of iodine to form 0. 1 mole each of the product. On mixing the two liquids, the sum of their initial volumes is equal to the volume of the final mixture. Hence, the correct option is a was this answer helpful? It has been found that gaseous iodine molecule just dissociates into iodine atoms after absorption of light at wavelengths 4995 a. At equilibrium, 40 percent of water (by mass) reacts with carbon monoxide according to the … (round off to one decimel place) The mole fraction of solute in the solution is 0. 2.
Is That Mole On Your Testicle Dangerous?
The vapour pressure of a solvent decreased by 10 mm of h g when a non-volatile solute was added to the solvent. What would be...